Pre-existing neutralizing antibodies can limit who is eligible for AAV9 gene therapy and complicate re-dosing. Teams need structural evidence to see where strong antibodies bind and how to redesign the capsid with lower visibility.
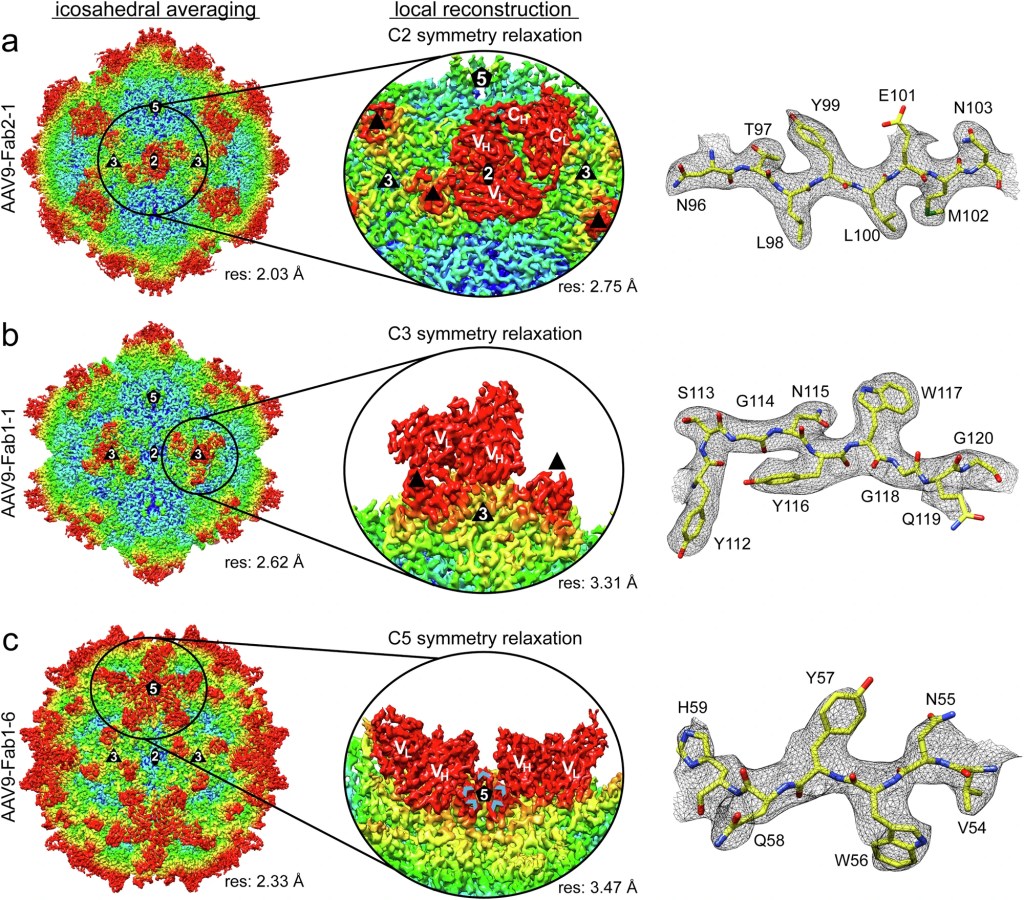
Figure from Mietzsch et al. (2025). License CC BY 4.0
What we studied
We analyzed human-derived neutralizing antibodies from patients treated with the AAV9 vector using high-resolution cryo-EM and localized reconstructions to capture non-icosahedral Fab footprints.
Key results
- Antibody binding clustered at the 2-fold depression and the 3-fold protrusions of the capsid, requiring localized refinement beyond global symmetry.
- Multiple epitopes partially overlap, explaining strong neutralization and cross-competition patterns observed across patient-derived antibodies.
- Structure-guided loop edits are proposed that aim to reduce antibody visibility while preserving manufacturability and biodistribution properties.
Why it matters
If your program faces screen failures due to anti-AAV9 antibodies, epitope mapping with localized reconstruction provides a shortlist of low-visibility variant ideas before costly in vivo work. This can expand patient eligibility and improve the odds of successful re-dosing.
Methods in brief
Single-particle cryo-EM, focused classification and localized reconstruction to resolve asymmetric Fab–capsid interactions, model fitting, and epitope accessibility assessment.
References
Mietzsch, M., Hsi, J., Nelson, A.R. et al. Structural characterization of antibody-responses following Zolgensma treatment for AAV capsid engineering to expand patient cohorts. Nat Commun 16, 3731 (2025). https://doi.org/10.1038/s41467-025-59088-4
